I already talked about thermal deburring and its process parameters in previous posts but today I received a question:
If methane combusts completely with 2 parts of oxygen, why does thermal deburring use 3–4 parts of oxygen for every 1 part of methane?
Let’s explore the chemistry, engineering, and safety behind this counterintuitive approach.
Combustion Chemistry:
Balanced Reaction of methane (CH₄) with oxygen (O₂), Stoichiometric:
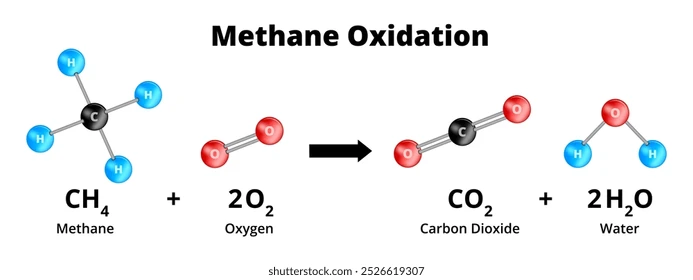
CH₄ + 2O₂ → CO₂ + 2H₂OThis means: 1 volume of methane reacts with 2 volumes of oxygen → producing carbon dioxide and water vapor.
Methane + Oxygen (2:1) → Complete Combustion
Why Use Excess Oxygen in Thermal Deburring?
0. Excess oxygen is used to oxidise the burrs!
1. Complete Combustion Under Fast Conditions
Thermal deburring happens in milliseconds. Perfect gas mixing is impossible in that short time. Extra oxygen guarantees that all methane molecules find oxygen to react with, minimizing:
- Carbon monoxide (CO)
- Unburned hydrocarbons
- Soot
2. Better Burr Removal
Excess oxygen promotes aggressive oxidation, helping to:
- Remove internal burrs (blind holes, slots)
- Create a uniform finish
3. Controlled Energy Release
A lean mixture (more oxygen than needed) results in:
- Lower peak pressure
- Slower flame speed
- Reduced risk of part damage
This helps protect both the components and the deburring chamber.
4. Cleaner Emissions
Excess oxygen ensures:
- Minimal carbon monoxide (CO)
- Lower volatile organic compounds (VOCs)
- Easier compliance with emission regulations
Stoichiometric vs Lean Combustion in Thermal Deburring
| Property | Stoichiometric (2:1) | Lean (4:1) |
|---|---|---|
| Flame Temperature | Very high | Slightly lower |
| Explosion Control | Less predictable | More stable |
| Burr Removal Quality | Good | Excellent |
| CO/CO₂ Emissions | Higher CO risk | Clean combustion |
| Safety | More explosive | Safer, less pressure |
Real-World Ratios in Practice
| Ratio (O₂:CH₄) | Use Case | Notes |
|---|---|---|
| 2:1 | Laboratory combustion | Stoichiometric, maximum heat |
| 3:1 – 4:1 | Thermal deburring (industry) | Lean mix, safer and more effective |
| >4:1 | Special cases or pressure control | Lower temp, but less energy |
Final Thoughts
Although textbooks say 2 parts oxygen is enough, real-world engineering requires more. In thermal deburring, using 3–4 parts oxygen per part methane improves:
- Safety
- Surface quality
- Emission control
- Process reliability
So next time someone asks why we “waste” oxygen in this process, we know that it’s not waste, it’s engineering 😉